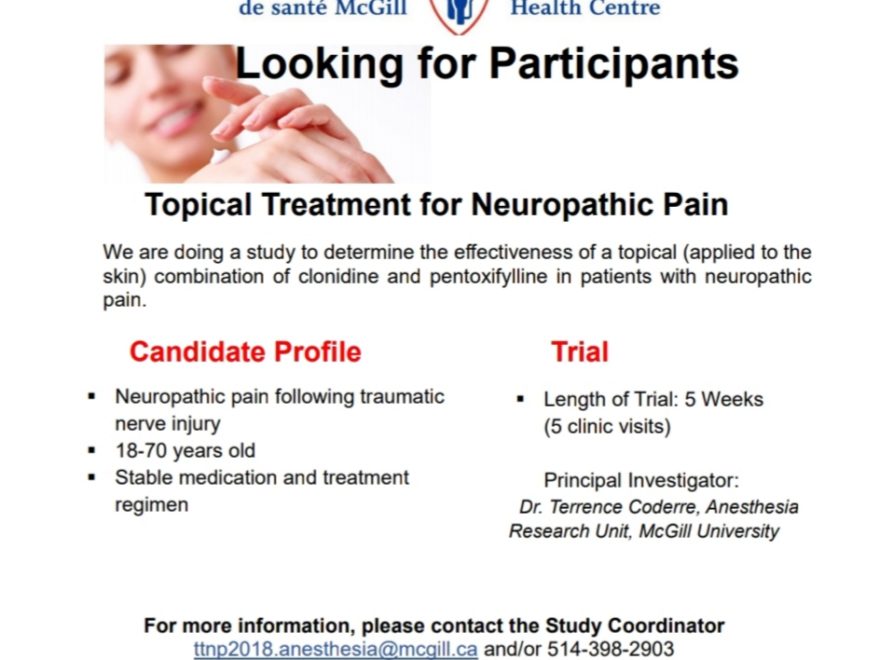
Back in October, I experienced something of a role reversal. After years of working in bioethics and managing a Research Ethics Board (REB), I was asked to consider participating in a clinical trial – as a patient. As a research participant.
If you’re in the US, a REB is the equivalent of an IRB; an Institutional Review Board. Whether it’s called an IRB or a REB, the purpose of these Boards is to protect research participants – the people who volunteer to take part in research.
Canada’s three principal governmental research funding agencies define human research participants (“participants” for short) as:
those individuals whose data, or responses to interventions, stimuli or questions by the researcher, are relevant to answering the research question.
Human participants are unique among the many parties involved in research, because they bear the primary risks of the research.
These individuals are often referred to as “research subjects”.
This Policy prefers the term “participant” because it better reflects the spirit behind the core principles: that individuals who choose to participate in research play a more active role than the term “subject” conveys.
As well, it reflects the range of research covered by this Policy, and the varied degree of involvement by participants “including the use of their data or human biological materials” that different types of research offer.
The core principles of this Policy “Respect for Persons, Concern for Welfare, and Justice” help to shape the relationship between researchers and participants.”(1)
There are many subtle differences between the Canadian and the US regulations governing clinical research, including the definitions used. For example, a different term is used to define a participant within the US research environment:
Human subject means an individual who is or becomes a participant in research, either as a recipient of the test article or as a control.
A subject may be either a healthy individual or a patient.” (2)
Differences also exist in how REBs and IRBs are defined. In the US, for example, an IRB is:
any board, committee, or other group formally designated by an institution to review, to approve the initiation of, and to conduct periodic review of, biomedical research involving human subjects.
The primary purpose of such review is to assure the protection of the rights and welfare of the human subjects.”(2)
Canada’s federal research funding agencies take a different approach, and define an REB as:
A body of researchers, community members, and others with specific expertise (e.g. in ethics, in relevant research disciplines) established by an institution to review the ethical acceptability of all research involving humans conducted within the institution’s jurisdiction or under its auspices.”(3)
I have a good grasp of how clinical trials function, and how important they are to creating better treatments for patients. So of course I said yes, when my specialist physician asked me whether I’d consider participating in a clinical trial!
He told me that if I was interested, a research coordinator would contact me to discuss a new research study for my rare disease. When the research coordinator called, my first question wasn’t about the research protocol; it was on the inclusion and exclusion criteria.
I wanted to find out right away whether any of my current medications, or anything in my medical history, would exclude me from participating in the research.
After all my years of work in research ethics, I was excited that I might finally have an opportunity to participate in a clinical trial myself! But one of the exclusion criteria was anti-hypertensive (blood pressure) medications.
I’ve been taking this type of medication preventively for a few years now. Although my blood pressure is only slightly on the high side, my family physician and I decided to treat it aggressively because many women in my family have had heart disease at about my age.
But taking these medications would mean that I couldn’t participate in the clinical trial. “But,” I thought to myself, “what if I asked my doctor if I could stop taking this medication for a few months?”
That would not only allow me to take part in the research, but also to check whether I still needed these daily medications. Especially now that I have so many more prescriptions than I used to, because of CRPS.
I explained my idea to the research coordinator, and asked if I could let her know a few weeks later whether I could participate in the study. I’d need to make a non-urgent appointment with my doctor, to describe my idea to him. She agreed, so I went to see my family physician.
He was happy with my idea of trying a few weeks without my anti-hypertensive medications, to find out whether I still needed them. After I promised to monitor my blood pressure several times a day, even at work.
He knew that I have a portable blood pressure monitor, because I’d occasionally been completing hypertension diaries – or logs – for him ,-) We agreed on an upper limit to my blood pressure; any higher than that and I’d immediately start taking these medications again.
This three-week test, with me tracking my blood pressure every few hours, went well. So today my family doctor gave me the go-ahead to be a research participant in the clinical trial for CRPS.
I just sent a message to the research coordinator, and am waiting to hear back from her. Maybe they’ve already recruited all the patients they need for this trial, but at least now I’ll know that I tried to be part of a research study. To potentially help other people struggling with the same disease.
By the way, I’m not getting paid or receiving any benefit to promote this research! I offered to share information about it, because I know how important it is to find viable treatments for patients suffering with CRPS. The
operational criteria to determine which patient may profit from what treatment are absent, and sound research data on the effectiveness of pharmacologic treatment in CRPS… are lacking.”(4)
This blog has always been, and remains, entirely non-commercial; I’m not promoting specific treatments or research. I’m simply writing about my own patient research journey, from the perspective of someone who has worked on the research from the bioethics perspective.
As always, thanks so much for popping in! Have a lovely day, and wish me luck – I’m really hoping to be able to participate in this research project ‘-) Stay tuned for news.
References
(1) The Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Social Sciences and Humanities Research Council of Canada (SSHRC). Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans, 2nd edition (TCPS 2). Chapter 2, Scope and Approach. 12 Oct 2017. Accessed 05 Jan 2019. Web:
http://www.pre.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/chapter2-chapitre2
(2) United States Government, Food and Drug Administration, Department of Health and Human Services. Code of Federal Regulations, Title 21, Volume 1 (21CFR56), Chapter 1, Subchapter A – General; Part 56 – Institutional Review Boards; Section 56.102 – Definitions. Rev 01 Apr 2018. Accessed 05 Jan 2019. Web:
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=56&showFR=1&subpartNode=21:1.0.1.1.22.1
(3) The Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Social Sciences and Humanities Research Council of Canada (SSHRC). Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans, 2nd edition (TCPS 2). Glossary. 10 Jun 2016. Accessed 05 Jan 2019. Web:
http://www.pre.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/glossary-glossaire/
(4) Gerard M. Ribbers, Alexander C. Geurts, Henk J. Stam, and Theo Mulder. Pharmacologic Treatment of Complex Regional Pain Syndrome I: A Conceptual Framework. Clinical Implications of Basic Research. Arch Phys Med Rehabil 2003;84: 141-6. Jan 2003. Accessed 05 Jan 2019. Web (PDF):
https://www.archives-pmr.org/article/S0003-9993(02)04894-3/pdf